
In this context, in vitro as well as in vivo experimental models have been developed to evaluate angiogenesis features, to screen a variety of new angiostatic molecules and to study their properties 7, 8. Other fields of interest, such as stem cell research, also require the evaluation of angiogenic capacities of EC progenitors as well as quantitative tools to monitor their proliferation and differentiation capacities 3, 6.
For example, the use of anti-angiogenic molecules still represents a promising therapeutic strategy to impede the development of solid tumors 4, 5.
#Imagej software angiogenesis full
Accurate and objective evaluation of these phenomena is necessary for the full comprehension of this process, for both fundamental approaches and pharmacological applications.
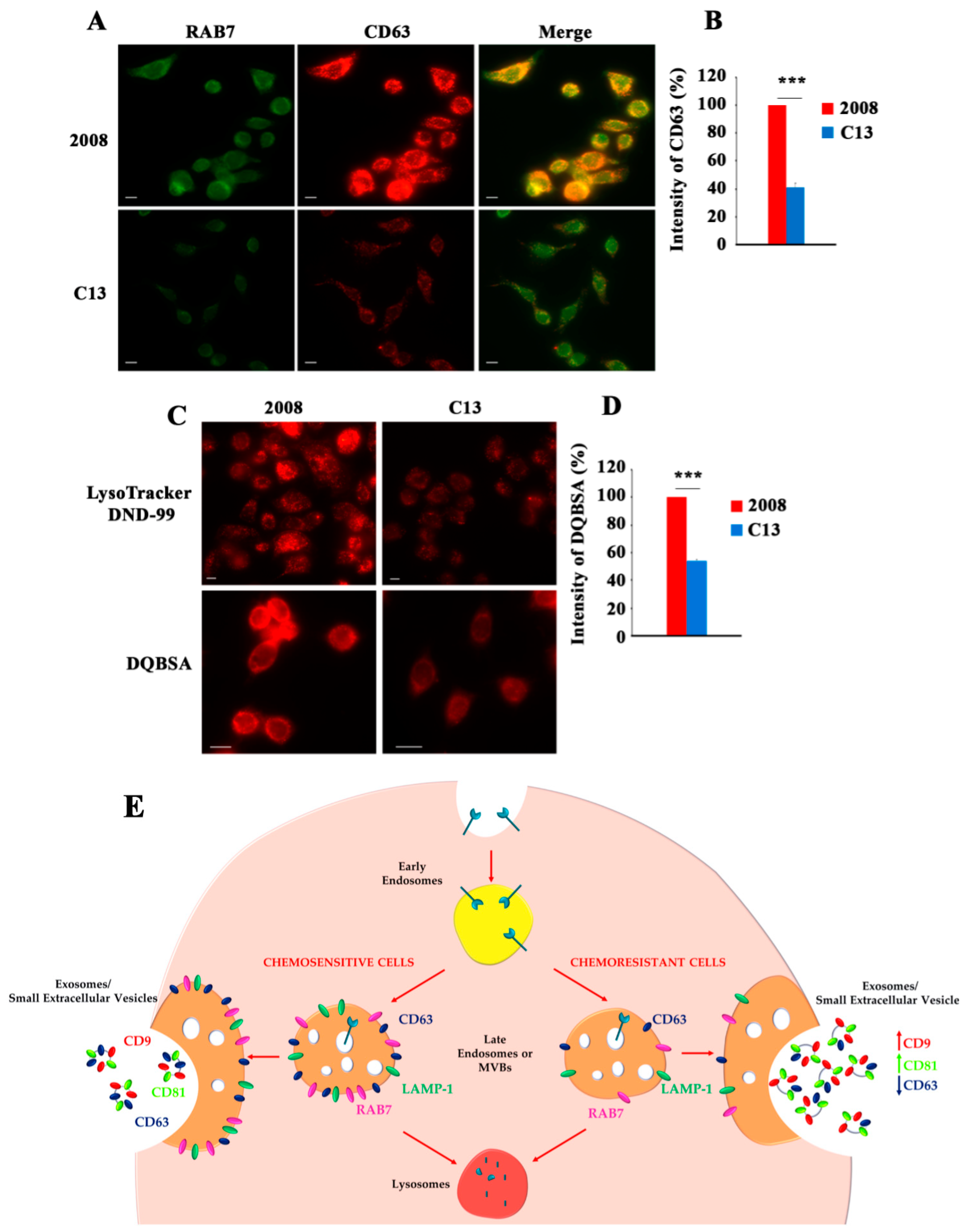
Under appropriate stimuli, these cells sprout from a root vessel, migrate, proliferate, then align and ultimately form tubes 2, 3. Endothelial cells (EC) represent the main cell type engaged in this process. Although the two methods do not assess the same biological step, our data suggest that they display specific and complementary information on the angiogenesis processes analysis.Īngiogenesis, the growth of new blood vessels from pre-existing ones, is a complex and critical process that takes place during vertebrate development, in specific physiological conditions in adult individuals and during different pathologies 1, 2. ETFA and FBA) in their efficiency, accuracy and statistical relevance to model angiogenesis patterns of Human Umbilical Vein EC (HUVEC). We detailed these two algorithms and used the new AA version to compare both methods ( i.e. Such a method is presented for the first time in fully automated mode and using non-destructive image acquisition. In this work, we developed and implemented a new algorithm for AA able to recognize microspheres and to analyze the attached capillary-like structures from the FBA model. We previously developed the “Angiogenesis Analyzer” for ImageJ (AA), a tool allowing analysis of ETFA-derived images, according to characteristics of the pseudo-capillary networks. The analytical evaluation of these two widely used assays still remains challenging in terms of observation method and image analysis. An alternative approach to ETFA is the “Fibrin Bead Assay” (FBA), based on the use of Cytodex 3 microspheres, which promote the growth of 3D capillary-like patterns from coated EC, suitable for high throughput in vitro angiogenesis studies. In suitable culture conditions, EC form two-dimensional (2D) branched structures that can lead to a meshed pseudo-capillary network. Among the different methods applied to study angiogenesis, the most commonly used is the “Endothelial Tube Formation Assay” (ETFA). This reproducible ex vivo choroidal sprouting assay can be used to assess compounds for potential treatment and for microvascular disease research to assess pathways involved in choroidal micro vessel proliferation using wild type and genetically modified mouse tissue.Angiogenesis assays based on in vitro capillary-like growth of endothelial cells (EC) are widely used, either to evaluate the effect of anti- and pro-angiogenesis drugs of interest, or to test and compare the functional capacities of various types of EC and progenitor cells. The images of individual choroid explant are taken with an inverted phase microscope and the sprouting area is quantified using a semi-automated macro plug-in to the ImageJ software developed in this lab. Medium is changed every other day and choroid sprouting is quantified at day 6. Mouse RPE/choroid/scleral explants are isolated and incubated in growth-factor-reduced basal membrane extract (BME) (day 0). This model includes the interaction between choroid vasculature (EC, macrophages, pericytes) and retinal pigment epithelium (RPE). A highly reproducible ex vivo choroidal sprouting assay as a model of choroidal microvascular proliferation was developed. However, isolating pure murine retinal endothelial cells is technically challenging and retinal ECs may have different proliferation responses than choroidal endothelial cells and different cell/cell interactions. Endothelial cell (EC) proliferation assays using human retinal microvascular endothelial cells (HRMECs) or isolated primary retinal ECs are widely used in vitro models to study retinal angiogenesis. Molecular Bases of Eyes Diseases Training Programĭate Published:2020 Aug 06 Abstract:Pathological choroidal angiogenesis, a salient feature of age-related macular degeneration, leads to vision impairment and blindness.

International Research & Training Program.K12 Clinician Scientist Training Program.Research Seminars, Conferences, and Symposia.Mobility Enhancement & Vision Rehabilitation.


 0 kommentar(er)
0 kommentar(er)
